Since the issuance by the United States Supreme Court of its opinion in Alice Corporation Pty Ltd. v. CLS Bank International, 573 U.S. 208, 134 S. Ct. 2347 (2014), the United States Patent...
Declaration made under Article 8(7)(a) of the Madrid Protocol: Malaysia
The Government of Malaysia deposited with WIPO the declaration indicating that Malaysia wishes to receive an individual fee...
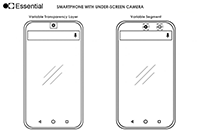
Back in the last year, we’ve seen the advent of waterdrop notches to fix the design crime made by Apple and its original iPhone X in 2017. This year, however, it was time for the pop-up camera...
ARISE+ IPR, in coordination with the national Intellectual Property (IP) offices of the ASEAN Member States, publishes a booklet on ASEAN Geographical Indications (GIs) procedures and products.
GIs...
Ericsson confirmed to IAM that Xiaomi and Ericsson have signed a global patent license agreement, and the two sides ‘shook hands’ at the Delhi High Court in India. So far, the patent litigation...
Page 17 of 47